Photochromic Torsional Switch (PTS): a light-driven actuator for the dynamic tuning of π-conjugation extension†
Abstract
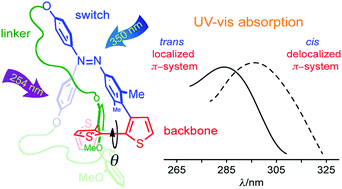
Here we present a molecular architecture that can reversibly change the geometric conformation of its π-system backbone via irradiation with two different wavelengths. The proposed ‘molecular actuator’ consists of a photoswitchable azobenzene orthogonally connected to a π-conjugated bithiophene by both direct and aliphatic linker-assisted bonding. Upon exposure to 350 nm light, the trans azobenzene moiety isomerizes to its cis form, causing the bithiophene to assume a semiplanar anti conformation (extended π-conjugation). Exposure to 254 nm light promotes the isomerization of the azobenzene unit back to its initial extended trans conformation, thus forcing the bithiophene fragment to twist out of coplanarity (restricted π-conjugation). The molecular conformation of the bithiophene was characterized using steady-state UV-vis and nuclear magnetic resonance spectroscopy, as well as ab initio computations. The proposed molecular design could be envisaged as a π-conjugation modulator, which has potential to be incorporated into extended linear π-systems, i.e. via the terminal α-thiophene positions, and used to tune their optical and electronic properties.

 Please wait while we load your content...
Please wait while we load your content...