Unified synthesis of mono/bis-arylated phenols via RhIII-catalyzed dehydrogenative coupling†
Abstract
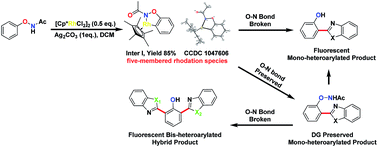
2,6-Bis-arylated phenols are rarely reported and are synthetically challenging. Directed C–H functionalization reactions, using a directing group (DG), might provide a convenient solution to their synthesis. However, this strategy usually results in partial cleavage of the directing group, preventing further/second C–H activation cascades. Herein we report a general strategy that allows for the precise control of the oxidation pathways so that directing groups can be either preserved or cleaved. We found that N-phenoxyacetamides could undergo ortho-arylation reactions with or without an external oxidant, yielding products with different oxidation states, notably the rare bis-arylated phenols. Notably, a unique rhodacycle intermediate was isolated, characterized by X-ray crystallography, and confirmed to be an active catalyst. Switching between internal and external oxidation could be a general strategy in diverse directed C–H functionalization reactions to realize bis-functionalized products.


 Please wait while we load your content...
Please wait while we load your content...