Reactivity of the copper(iii)-hydroxide unit with phenols†
Abstract
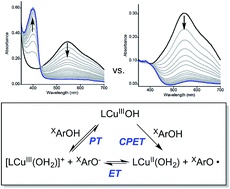
Kinetic studies of the reactions of two previously characterized copper(III)-hydroxide complexes (LCuOH and NO2LCuOH, where L = N,N′-bis(2,6-diisopropylphenyl)-2,6-pyridine-dicarboxamide and NO2L = N,N′-bis(2,6-diisopropyl-4-nitrophenyl)pyridine-2,6-dicarboxamide) with a series of para substituted phenols (XArOH where X = NMe2, OMe, Me, H, Cl, NO2, or CF3) were performed using low temperature stopped-flow UV-vis spectroscopy. Second-order rate constants (k) were determined from pseudo first-order and stoichiometric experiments, and follow the trends CF3 < NO2 < Cl < H < Me < OMe < NMe2 and LCuOH < NO2LCuOH. The data support a concerted proton–electron transfer (CPET) mechanism for all but the most acidic phenols (X = NO2 and CF3), for which a more complicated mechanism is proposed. For the case of the reactions between NO2ArOH and LCuOH in particular, competition between a CPET pathway and one involving initial proton transfer followed by electron transfer (PT/ET) is supported by multiwavelength global analysis of the kinetic data, formation of the phenoxide NO2ArO− as a reaction product, observation of an intermediate [LCu(OH2)]+ species derived from proton transfer from NO2ArOH to LCuOH, and thermodynamic arguments indicating that initial PT should be competitive with CPET.


 Please wait while we load your content...
Please wait while we load your content...