Synthesis of malhamensilipin A exploiting iterative epoxidation/chlorination: experimental and computational analysis of epoxide-derived chloronium ions†
Abstract
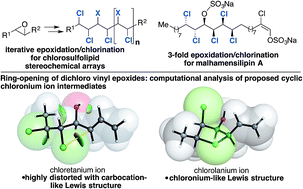
We report a 12-step catalytic enantioselective formal synthesis of malhamensilipin A (3) and diastereoisomeric analogues from (E)-2-undecenal. The convergent synthesis relied upon iterative epoxidation and phosphorus(V)-mediated deoxydichlorination reactions as well a titanium-mediated epoxide-opening to construct the C11–C16 stereohexad. The latter transformation occurred with very high levels of stereoretention regardless of the C13 configuration of the parent epoxide, implicating anchimeric assistance of either the γ- or δ-chlorine atoms, and the formation of chloretanium or chlorolanium ions, respectively. A computational analysis of the chloronium ion intermediates provided support for the involvement of chlorolanium ions, whereas the potential chloretanium ions were found to be less likely intermediates on the basis of their greater carbocationic character.


 Please wait while we load your content...
Please wait while we load your content...