Proton-coupled electron transfer in the electrocatalysis of CO2 reduction: prediction of sequential vs. concerted pathways using DFT†
Abstract
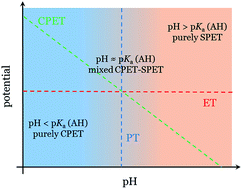
Herein we investigate computationally in detail the mechanism of the formation of the carboxylate adduct during the electroreduction of CO2 in water catalysed by cobalt porphyrin complexes. Specifically, we address qualitatively the competition between the concerted and sequential pathways for the proton-coupled electron transfer. We use a simple methodology for accurate computation of the pKa of the neutral and anionic carboxylate intermediates, [CoP–COOH] and [CoP–COOH]− (where CoP is a cobalt porphine complex), based on the isodesmic proton-exchange reaction scheme. The predicted values are used as in input for a theoretical model that describes the transition between the sequential and concerted pathways. The activation of the sequential pathway (ET–PT) that leads to the formation of the neutral [CoP–COOH] intermediate at pH ≈ 3.5 (pKa[CoP–COOH] = 3.5 ± 0.4), as predicted by the calculations, is in good agreement with the drastic increase in the faradaic efficiency of the CO2 reduction reaction towards CO at pH = 3 compared to pH = 1, as experimentally observed. This confirms the existence of the CO2 anionic adduct [CoP–CO2]− as a viable intermediate at pH = 3 and its crucial role for the pH dependence of the faradaic efficiency for the CO2 reduction. The analysis also shows that when the pH is significantly higher than the pKa of the neutral carboxylate adduct, the CO2 reduction has to go through an alternative pathway with the formation of the anionic carboxylate intermediate [CoP–COOH]−. It is formed through a concerted proton–electron transfer step from the anionic CO2 adduct [CoP–CO2]− when the pH is below ∼8.6 (pKa[CoP–COOH]− = 8.6 ± 0.4). At pH ≈ 8.6 and above, another decoupled ET–PT is predicted to take place, leading to the formation of a dianionic CO2 adduct [CoP–CO2]2−.
- This article is part of the themed collection: Global Energy Challenges: Hydrogen Energy

 Please wait while we load your content...
Please wait while we load your content...