Micro-flow photosynthesis of new dienophiles for inverse-electron-demand Diels–Alder reactions. Potential applications for pretargeted in vivo PET imaging†
Abstract
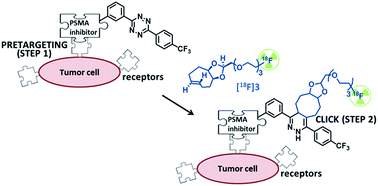
Pretargeted PET imaging has emerged as an effective two-step in vivo approach that combines the superior affinity and selectivity of antibodies with the rapid pharmacokinetics and favorable dosimetry of smaller molecules radiolabeled with short-lived radionuclides. This approach can be based on the bioorthogonal inverse-electron-demand Diels–Alder (IEDDA) reaction between tetrazines and trans-cyclooctene (TCO) derivatives. We aimed to develop new [18F]TCO–dienophiles with high reactivity for IEDDA reactions, and favorable in vivo stability and pharmacokinetics. New dienophiles were synthesized using an innovative micro-flow photochemistry process, and their reaction kinetics with a tetrazine were determined. In vivo stability and biodistribution of the most promising 18F-radiolabeled-TCO-derivative ([18F]3) was investigated, and its potential for in vivo pretargeted PET imaging was assessed in tumor-bearing mice. We demonstrated that [18F]3 is a suitable dienophile for IEDDA reactions and for pretargeting applications.


 Please wait while we load your content...
Please wait while we load your content...