Enantioselective bifunctional iminophosphorane catalyzed sulfa-Michael addition of alkyl thiols to unactivated β-substituted-α,β-unsaturated esters†
Abstract
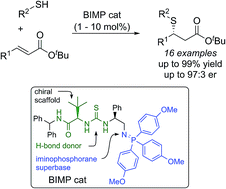
The highly enantioselective sulfa-Michael addition of alkyl thiols to unactivated β-substituted-α,β-unsaturated esters catalyzed by a bifunctional iminophosphorane (BIMP) organocatalyst is described. The low acidity of the alkyl thiol pro-nucleophiles is overcome by the high Brønsted basicity of the catalyst and the chiral scaffold/thiourea hydrogen-bond donor moiety provides the required enantiofacial discrimination in the addition step. The reaction is broad in scope with respect to the alkyl thiol and β-substituent of the α,β-unsaturated ester, affords sulfa-Michael adducts in excellent yields (up to >99%) and enantioselectivity (up to 97 : 3 er) and can operate down to 1 mol% catalyst loading.
- This article is part of the themed collection: ISACS19: Challenges in Organic Chemistry

 Please wait while we load your content...
Please wait while we load your content...