Thioester reduction and aldehyde transamination are universal steps in actinobacterial polyketide alkaloid biosynthesis†
Abstract
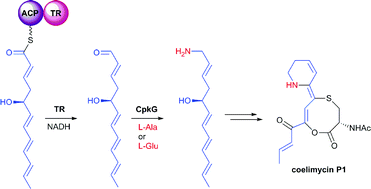
Actinobacteria produce a variety of polyketide alkaloids with unusual structures. Recently, it was shown that a type I modular polyketide synthase (PKS) is involved in the assembly of coelimycin P1, a polyketide alkaloid produced by Streptomyces coelicolor M145. However, the mechanisms for converting the product of the PKS to coelimycin P1 remain to be elucidated. Here we show that the C-terminal thioester reductase (TR) domain of the PKS and an ω-transaminase are responsible for release of the polyketide chain as an aldehyde and its subsequent reductive amination. Bioinformatics analyses identified numerous gene clusters in actinobacterial genomes that encode modular PKSs with a C-terminal TR domain and a homolog of the ω-transaminase. These are predicted to direct the biosynthesis of both known and novel polyketide alkaloids, suggesting that reductive chain release and transamination constitutes a conserved mechanism for the biosynthesis of such metabolites.
- This article is part of the themed collection: Celebrating the 2017 RSC Prize and Award Winners


 Please wait while we load your content...
Please wait while we load your content...