The dual role of thiourea in the thiotrifluoromethylation of alkenes†
Abstract
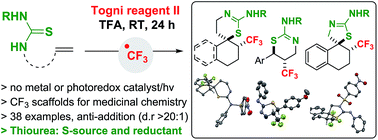
Alkenes substituted with a thiourea undergo C–CF3 followed by intramolecular C–S bond formation with the Togni reagent and trifluoroacetic acid (TFA) at room temperature; thiols and thioamides are not suitable S-sources for this reaction. This anti-addition process involves a CF3 radical, and affords CF3-substituted thiazolines and thiazines for medicinal applications. A metal or photoredox catalyst is not required as the thiourea acts as a reductant, as well as serving as an S-source capable of adding to a C-centered radical. Mechanistic work comparing the reactivity of thiourea, urea, thioamide and thiol in the context of alkene trifluoromethylation demonstrates that in this series, the thiourea is unique for its ability to release CF3 radical from the Togni reagent, and to orchestrate trifluoromethylation followed by S-cyclization with both activated and unactivated alkenes.
- This article is part of the themed collection: Celebrating our 2019 Prize and Award winners

 Please wait while we load your content...
Please wait while we load your content...