Sizing the role of London dispersion in the dissociation of all-meta tert-butyl hexaphenylethane†
Abstract
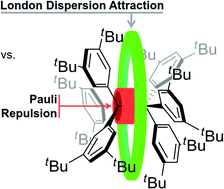
The structure and dynamics of enigmatic hexa(3,5-di-tert-butylphenyl)ethane was characterized via NMR spectroscopy for the first time. Our variable temperature NMR analysis demonstrates an enthalpy–entropy compensation that results in a vanishingly low dissociation energy (ΔG298d = −1.60(6) kcal mol−1). An in silico study of increasingly larger all-meta alkyl substituted hexaphenylethane derivatives (Me, iPr, tBu, Cy, 1-Ad) reveals a non-intuitive correlation between increased dimer stability with increasing steric crowding. This stabilization originates from London dispersion as expressed through the increasing polarizability of the alkyl substituents. Substitution with conformationally flexible hydrocarbon moieties, e.g., cyclohexyl, introduces large unfavourable entropy contributions. Therefore, using rigid alkyl groups like tert-butyl or adamantyl as dispersion energy donors (DED) is essential to help stabilize extraordinary bonding situations.


 Please wait while we load your content...
Please wait while we load your content...