Highly planar diarylamine-fused porphyrins and their remarkably stable radical cations†
Abstract
Oxidative fusion reactions of meso-phenoxazino Ni(II) porphyrin were found to be temperature dependent, giving rise to either a doubly phenylene-fused product at room temperature or a singly phenoxazine-fused product at 70 °C. The latter was further oxidized to a doubly phenoxazine-fused Ni(II) porphyrin, which was subsequently converted to the corresponding free base porphyrin and Zn(II) porphyrin. Compared to previously reported diphenylamine-fused porphyrins that displayed a molecular twist, doubly phenoxazine-fused porphyrins exhibited distinctly different properties owing to their highly planar structures, such as larger fluorescence quantum yields, formation of an offset face-to-face dimer both in solution and the solid state, and the generation of a mixed-valence π-radical cation dimer upon electrochemical oxidation. One-electron oxidation of the phenoxazine-fused Ni(II) porphyrin with Magic Blue gave the corresponding radical cation, which was certainly stable and could be isolated by separation over a silica gel column but slowly chlorinated at the reactive β-positions in the solid state. This finding led to us to examine β,β′-dichlorinated phenoxazine-fused and diphenylamine-fused Ni(II) porphyrins, which, upon treatment with Magic Blue, provided remarkably stable radical cations to an unprecedented level. It is actually possible to purify these radical cations by silica gel chromatography, and they can be stored for over 6 months without any sign of deterioration. Moreover, they exhibited no degradation even after the CH2Cl2 solution was washed with water. However, subtle structural differences (planar versus partly twisted) led to different crystal packing structures and solid-state magnetic properties.
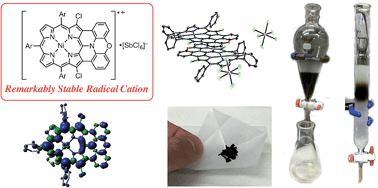
- This article is part of the themed collection: International Symposium on Macrocyclic & Supramolecular Chemistry (ISMSC) in conjunction with ISACS

 Please wait while we load your content...
Please wait while we load your content...