Greigite Fe3S4 as a new anode material for high-performance sodium-ion batteries†
Abstract
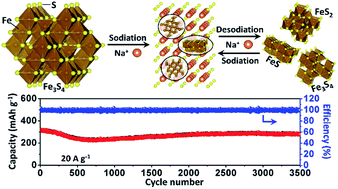
Transition metal dichalcogenide materials have been considered as promising anode materials for rechargeable sodium-ion batteries because of their high specific capacity and low cost. Here, we demonstrate an iron sulfide Fe3S4 as a new anode material for a rechargeable sodium-ion battery. The involved conversion mechanism has been proved when the as-prepared Fe3S4 was used as the host material for sodium storage. Remarkably, a compound FeSx with quantum size generated by conversion reaction overcame the kinetic and thermodynamic constraints of chemical conversion to achieve superior cycling and rate capability. As a result, the as-prepared Fe3S4 electrode delivers a high reversible specific capacity of 548 mA h g−1 at 0.2 A g−1, together with an excellent cycling stability of 275 mA h g−1 after 3500 cycles at 20 A g−1.
- This article is part of the themed collection: Global Energy Challenges: Electrochemical Energy


 Please wait while we load your content...
Please wait while we load your content...