Effect of zinc cations on the kinetics of supramolecular assembly and the chirality of porphyrin J-aggregates†
Abstract
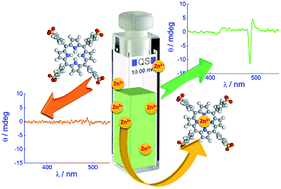
Dilute aqueous solutions of anionic meso-4-sulfonatophenyl-porphyrin (TPPS) extract zinc(II) ions from glass or quartz surfaces at room temperature and efficiently form the corresponding metal complex (ZnTPPS). The partial or complete formation of ZnTPPS has been probed by UV/Vis spectroscopy and both static and time-resolved fluorescence. The source of zinc(II) ions has been clearly identified through inductively coupled plasma optical emission spectrometry. The presence of increasing amounts of ZnTPPS slows down the rate of TPPS J-aggregate formation in acid solution. This influences the nucleation step and has a profound impact on the onset of chirality in these species. This evidence indicates the important role of this adventitious metal ion in the interpretation of various spectroscopic and kinetic data for the self-assembly of the TPPS porphyrin and provides some insights into controversial findings on their chirality. The use of this metal derivative as the starting compound for in situ formation of monomeric TPPS is suggested.


 Please wait while we load your content...
Please wait while we load your content...