Copper-catalyzed dehydrogenative borylation of terminal alkynes with pinacolborane†
Abstract
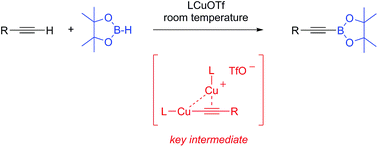
LCuOTf complexes [L = cyclic (alkyl)(amino)carbenes (CAACs) or N-heterocyclic carbenes (NHCs)] selectively promote the dehydrogenative borylation of C(sp)–H bonds at room temperature. It is shown that σ,π-bis(copper) acetylide and copper hydride complexes are the key catalytic species.

 Please wait while we load your content...
Please wait while we load your content...