Enantiodivergent Steglich rearrangement of O-carboxylazlactones catalyzed by a chirality switchable helicene containing a 4-aminopyridine unit†
Abstract
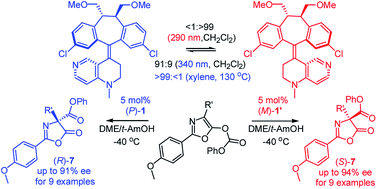
A pseudo-enantiomeric pair of optically switchable helicenes containing a catalytic 4-N-methylaminopyridine (MAP) bottom unit and a C2-symmetric, (10R,11R)-dimethoxymethyl-dibenzosuberane top template was synthesized. They underwent complementary photoswitching at 290 nm (P/M′, <1/>99) and 340 nm (P/M′, 91/9) and unidirectional thermo-rotation at 130 °C (P/M′, >99/<1). They were utilized to catalyze enantiodivergent Steglich rearrangement of O- to C-carboxylazlactones, with formation of either enantiomer with up to 91% ee (R) and 94% ee (S), respectively.

 Please wait while we load your content...
Please wait while we load your content...