Imaging specific newly synthesized proteins within cells by fluorescence resonance energy transfer†
Abstract
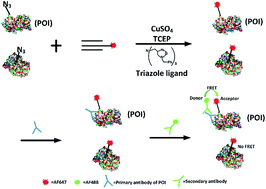
Metabolic azide amino acid labelling followed by the use of bioorthogonal chemistry is an efficient technique for imaging newly synthesized proteins. Recently, AHA-labelling together with the proximity-ligation assay was used to identify newly synthesized proteins of interest (POI) (Tom Dieck et al., Nat. Meth. 2015, 12, 411). Here we build on this study replacing the proximity-ligation assay with FRET to improve the spatial resolution. Herein, we develop a FRET-based strategy for imaging the newly synthesized endogenous POI within cells: a FRET acceptor is installed onto the newly synthesized proteins via click chemistry, and a FRET donor onto the POI via immunocytochemistry. We found that a photobleaching based FRET efficiency imaging mode and a fluorescence lifetime imaging mode showed the distribution of newly synthesized proteins more accurately compared to the direct observation of FRET signals. We demonstrated the capability of this FRET-based imaging method by visualizing several newly synthesized proteins including TDP-43, tubulin and CaMKIIα in different cell lines. This novel analytical imaging method could be used to visualize other specific endogenous proteins of interest in situ.

 Please wait while we load your content...
Please wait while we load your content...