Heptamethylindenyl (Ind*) enables diastereoselective benzamidation of cyclopropenes via Rh(iii)-catalyzed C–H activation†
Abstract
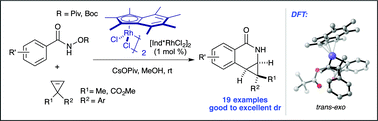
The diastereoselective coupling of O-substituted arylhydroxamates and cyclopropenes mediated by Rh(III) catalysis was successfully developed. Through ligand development, the diastereoselectivity of this reaction was improved using a heptamethylindenyl (Ind*) ligand, which has been rationalized using quantum chemical calculations. In addition, the nature of the O-substituted ester of benzhydroxamic acid proved important for high diastereoselectivity. This transformation tolerates a variety of benzamides and cyclopropenes that furnish cyclopropa[c]dihydroisoquinolones with high diastereocontrol, which could then be easily transformed into synthetically useful building blocks for pharmaceuticals and bio-active molecules.


 Please wait while we load your content...
Please wait while we load your content...