Sequence-defined oligo(ortho-arylene) foldamers derived from the benzannulation of ortho(arylene ethynylene)s†
Abstract
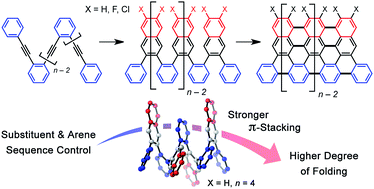
A Cu-catalyzed benzannulation reaction transforms ortho(arylene ethynylene) oligomers into ortho-arylenes. This approach circumvents iterative Suzuki cross-coupling reactions previously used to assemble hindered ortho-arylene backbones. These derivatives form helical folded structures in the solid-state and in solution, as demonstrated by X-ray crystallography and solution-state NMR analysis. DFT calculations of misfolded conformations are correlated with variable-temperature 1H and EXSY NMR to reveal that folding is cooperative and more favorable in halide-substituted naphthalenes. Helical ortho-arylene foldamers with specific aromatic sequences organize functional π-electron systems into arrangements ideal for ambipolar charge transport and show preliminary promise for the surface-mediated synthesis of structurally defined graphene nanoribbons.


 Please wait while we load your content...
Please wait while we load your content...