Antiangiogenic platinum through glycan targeting†
Abstract
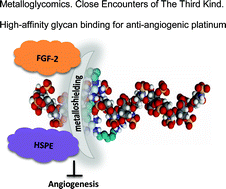
Heparan sulfate is identified as a ligand receptor for polynuclear platinum anti-cancer agents through sulfate cluster binding. We present a new biological role for platinum and coordination compounds and a new target for metal-based drugs while presenting a new chemotype for heparanase and growth factor inhibition through modulation (metalloshielding) of their interactions. Masking of extracellular (ECM)-resident heparan sulfate (HS) through metalloshielding results in very effective inhibition of physiologically critical HS functions including enzyme (heparanase, HPSE) and protein growth factor recognition. The interaction of the highly cationic polynuclear platinum complexes (PPCs) with the highly sulfated pentasaccharide Fondaparinux (FPX, in this case as a model HS-like substrate) results in inhibition of its cleavage by the HS-related enzyme heparanase. Binding of the fibroblast growth factor FGF-2 to HS is also inhibited with consequences for downstream signalling events as measured by a reduction in accumulation of phospho-S6 ribosomal protein in human colon tumor HCT-116 cells. The end-point of inhibition of HPSE activity and growth factor growth factor signaling is the prevention of cell invasion and angiogenesis. Finally these events culminate in inhibition of HCT-116 cell invasion at sub-cytotoxic concentrations and the process of angiogenesis. A competition assay shows that Fondaparinux can sequester the 8+ TriplatinNC from bound DNA, emphasising the strength of PPC–HS interactions. Altering the profile of platinum agents from cytotoxic to anti-metastatic has profound implications for future directions in the development of platinum-based chemotherapeutics.


 Please wait while we load your content...
Please wait while we load your content...