Photodriven hydrogen evolution by molecular catalysts using Al2O3-protected perylene-3,4-dicarboximide on NiO electrodes†
Abstract
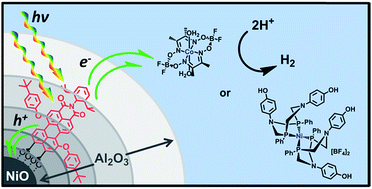
The design of efficient hydrogen-evolving photocathodes for dye-sensitized photoelectrochemical cells (DSPECs) requires the incorporation of molecular light absorbing chromophores that are capable of delivering reducing equivalents to molecular proton reduction catalysts at rates exceeding those of charge recombination events. Here, we report the functionalization and kinetic analysis of a nanostructured NiO electrode with a modified perylene-3,4-dicarboximide chromophore (PMI) that is stabilized against degradation by atomic layer deposition (ALD) of thick insulating Al2O3 layers. Following photoinduced charge injection into NiO in high yield, films with Al2O3 layers demonstrate longer charge separated lifetimes as characterized via femtosecond transient absorption spectroscopy and photoelectrochemical techniques. The photoelectrochemical behavior of the electrodes in the presence of Co(II) and Ni(II) molecular proton reduction catalysts is examined, revealing reduction of both catalysts. Under prolonged irradiation, evolved H2 is directly observed by gas chromatography supporting the applicability of PMI embedded in Al2O3 as a photocathode architecture in DSPECs.
- This article is part of the themed collections: Celebrating the 2017 RSC Prize and Award Winners, Global Energy Challenges: Solar Energy and Global Energy Challenges: Energy Applications

 Please wait while we load your content...
Please wait while we load your content...