Design and engineering of water-soluble light-harvesting protein maquettes†
Abstract
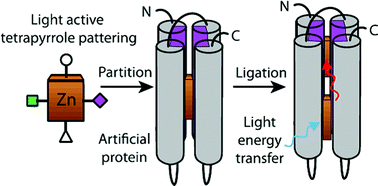
Natural selection in photosynthesis has engineered tetrapyrrole based, nanometer scale, light harvesting and energy capture in light-induced charge separation. By designing and creating nanometer scale artificial light harvesting and charge separating proteins, we have the opportunity to reengineer and overcome the limitations of natural selection to extend energy capture to new wavelengths and to tailor efficient systems that better meet human as opposed to cellular energetic needs. While tetrapyrrole cofactor incorporation in natural proteins is complex and often assisted by accessory proteins for cofactor transport and insertion, artificial protein functionalization relies on a practical understanding of the basic physical chemistry of protein and cofactors that drive nanometer scale self-assembly. Patterning and balancing of hydrophobic and hydrophilic tetrapyrrole substituents is critical to avoid natural or synthetic porphyrin and chlorin aggregation in aqueous media and speed cofactor partitioning into the non-polar core of a man-made water soluble protein designed according to elementary first principles of protein folding. This partitioning is followed by site-specific anchoring of tetrapyrroles to histidine ligands strategically placed for design control of rates and efficiencies of light energy and electron transfer while orienting at least one polar group towards the aqueous phase.


 Please wait while we load your content...
Please wait while we load your content...