N-heterocyclic carbene induced reductive coupling of phosphorus tribromide. Isolation of a bromine bridged P–P bond and its subsequent reactivity†
Abstract
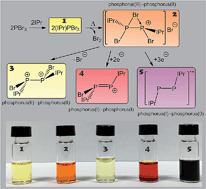
The room temperature reaction of a 1 : 1 mixture of phosphorus tribromide (PBr3) and the N-heterocyclic carbene 1,3-bis(2,6-diisopropylphenyl)-imidazol-2-ylidene (IPr) quantitatively affords the Lewis acid–base adduct (IPr)PBr3 (1). Interestingly, when 1 is heated between 55 and 65 °C for a period of several days, dark red crystals slowly begin to form in the reaction vessel accompanied by the release of bromine. The resulting crystalline sample, [P2(IPr)2Br3]Br ([2]Br), results from the reductive coupling of two equivalents of 1, and contains a cationic moiety with a P–P bond that is bridged by a bromine atom. Anion exchange reactions with Na[BArF4] (BArF4 = B(3,5-{CF3}2C6H3)4) afford [2][BArF4]. Abstraction of two equivalents of bromine allows for the isolation of the unprecedented dicationic species [P2(IPr)2Br2]2+ (3) which was isolated and structurally authenticated as two different [BArF4]− salts. Reaction of 2 with mild reductants such as SnBr2 or tetrakis(dimethylamino)ethylene (TDAE) affords [P2(IPr)2Br]+ (4) and the known radical cation [P2(IPr)2]˙+ (5), respectively. These studies show that relatively weak P–Br bonds present in compounds 1–4 can be cleaved in a straightforward manner to afford low oxidation state compounds in high yields.

 Please wait while we load your content...
Please wait while we load your content...