Converting disulfide bridges in native peptides to stable methylene thioacetals†
Abstract
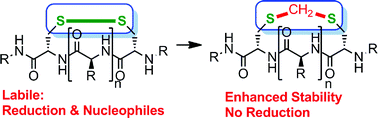
Disulfide bridges play a crucial role in defining and rigidifying the three-dimensional structure of peptides. However, disulfides are inherently unstable in reducing environments. Consequently, the development of strategies aiming to circumvent these deficiencies – ideally with little structural disturbance – are highly sought after. Herein, we report a simple protocol converting the disulfide bond of peptides into highly stable methylene thioacetal. The transformation occurs under mild, biocompatible conditions, enabling the conversion of unprotected native peptides into analogues with enhanced stability. The developed protocol is applicable to a range of peptides and selective in the presence of a multitude of potentially reactive functional groups. The thioacetal modification annihilates the reductive lability and increases the serum, pH and temperature stability of the important peptide hormone oxytocin. Moreover, it is shown that the biological activities for oxytocin are retained.

 Please wait while we load your content...
Please wait while we load your content...