Intramolecular 1,5-C(sp3)–H radical amination via Co(ii)-based metalloradical catalysis for five-membered cyclic sulfamides†
Abstract
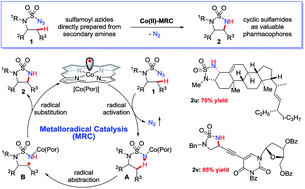
Co(II)-based metalloradical catalysis (MRC) proves effective for intramolecular 1,5-C–H amination of sulfamoyl azides under neutral and nonoxidative conditions, providing a straightforward approach to access strained 5-membered cyclic sulfamides with nitrogen gas as the only byproduct. The metalloradical amination system is applicable to different types of C(sp3)–H bonds and has a high degree of functional group tolerance. Additional features of the Co(II)-catalyzed 1,5-C–H amination include excellent chemoselectivity toward allylic and propargylic C–H bonds. The unique reactivity and selectivity profile of the Co(II)-catalyzed 1,5-C–H amination is attributed to the underlying radical mechanism of MRC.

 Please wait while we load your content...
Please wait while we load your content...