How covalence breaks adsorption-energy scaling relations and solvation restores them†
Abstract
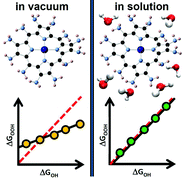
It is known that breaking the scaling relations between the adsorption energies of *O, *OH, and *OOH is paramount in catalyzing more efficiently the reduction of O2 in fuel cells and its evolution in electrolyzers. Taking metalloporphyrins as a case study, we evaluate here the adsorption energies of those adsorbates on the metal centers Cr, Mn, Fe, Co, Ni and Cu, using H, F, OH, NH2, CH3, and BH2 as ring ligands. We show that covalence systematically breaks scaling relations under vacuum by strengthening certain M–OOH bonds. However, covalence modifies adsorbate solvation in solution depending on the degree of covalence of the metal–adsorbate bonds. The two effects have similar magnitudes and opposite signs, such that scaling relations are restored in solution. Thus, solvation is a crucial ingredient that must be taken into account in studies aimed at breaking scaling relations in solution. Our findings suggest that the choice of metal and ligand determines the catalytic activity within the limits imposed by scaling relations, whereas the choice of an appropriate solvent can drive such activity beyond those limits.
- This article is part of the themed collection: Global Energy Challenges: Hydrogen Energy


 Please wait while we load your content...
Please wait while we load your content...