The GTPase hGBP1 converts GTP to GMP in two steps via proton shuttle mechanisms†
Abstract
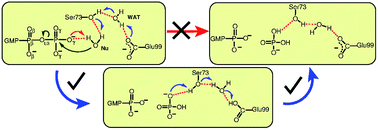
GTPases play a crucial role in the regulation of many biological processes by catalyzing the hydrolysis of GTP into GDP. The focus of this work is on the dynamin-related large GTPase human guanine nucleotide binding protein-1 (hGBP1) which is able to hydrolyze GTP even to GMP. Here, we studied the largely unknown mechanisms of both GTP and GDP hydrolysis steps utilizing accelerated ab initio QM/MM metadynamics simulations to compute multi-dimensional free energy landscapes. We find an indirect substrate-assisted catalysis (SAC) mechanism for GTP hydrolysis involving transfer of a proton from the water nucleophile to a nonbridging phosphoryl oxygen via a proton relay pathway where the rate-determining first step is concerted-dissociative nature. A “composite base” consisting of Ser73, Glu99, a bridging water molecule, and GTP was found to activate the nucleophilic water, thus disclosing the complex nature of the general base in hGBP1. A nearly two-fold reduction in the free energy barrier was obtained for GTP hydrolysis in the enzyme in comparison to bulk solvent. The subsequent GDP hydrolysis in hGBP1 was also found to follow a water-mediated proton shuttle mechanism. It is expected that the proton shuttle mechanisms unravelled for hGBP1 apply to many classes of GTPases/ATPases that possess an optimally-arranged hydrogen bonding network, which connects the catalytic water to a proton acceptor.

 Please wait while we load your content...
Please wait while we load your content...