Divergent ynamide reactivity in the presence of azides – an experimental and computational study†
Abstract
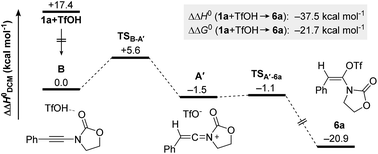
An unusually divergent reactivity of ynamides in the presence of azides is reported. This new keteniminium-based methodology, which only requires triflic acid as promoter, facilitates access to β-enaminoamides and biologically important oxazolidine-2,4-diones in a highly selective, divergent manner that is fully controllable by the present azide. A mechanistic rationale for these divergent reaction pathways is delineated and supported by extensive density functional theory analyses, as well as selected mechanistic experiments.

 Please wait while we load your content...
Please wait while we load your content...