Square channels formed by a peptide derived from transthyretin†
Abstract
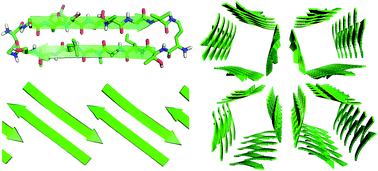
High-resolution structures of peptide supramolecular assemblies are key to understanding amyloid diseases and designing peptide-based materials. This paper explores the supramolecular assembly of a macrocyclic β-sheet peptide derived from transthyretin (TTR). The peptide mimics the β-hairpin formed by the β-strands G and H of TTR, which form the interface of the TTR tetramer. X-ray crystallography reveals that the peptide does not form a tetramer, but rather assembles to form square channels. The square channels are formed by extended networks of β-sheets and pack in a “tilted windows” pattern. This unexpected structure represents an emergent property of the peptide and broadens the scope of known supramolecular assemblies of β-sheets.
- This article is part of the themed collection: ISACS19: Challenges in Organic Chemistry

 Please wait while we load your content...
Please wait while we load your content...