Multi-electron reactivity of a cofacial di-tin(ii) cryptand: partial reduction of sulfur and selenium and reversible generation of S3˙−†
Abstract
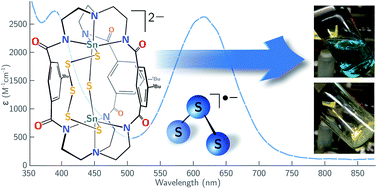
Cofacial bimetallic tin(II) ([Sn2(mBDCA-5t)]2−, 1) and lead(II) ([Pb2(mBDCA-5t)]2−, 2) complexes have been prepared by hexadeprotonation of hexacarboxamide cryptand mBDCA-5t-H6 together with double Sn(II) or Pb(II) insertion. Reaction of 1 with elemental sulfur or selenium generates di-tin polychalcogenide complexes containing μ-E and bridging μ-E5 ligands where E = S or Se, and the Sn(II) centers have both been oxidized to Sn(IV). Solution and solid-state UV-Vis spectra of [(μ-S5)Sn2(μ-S)(mBDCA-5t)]2− (4) indicate that the complex acts reversibly as a source of S3˙− in DMF solution with a Keq = 0.012 ± 0.002. Reductive removal of all six chalcogen atoms is achieved through treatment of [(μ-E5)Sn2(μ-E)(mBDCA-5t)]2− with PR3 (R = tBu, Ph, OiPr) to produce six equiv. of the corresponding EPR3 compound with regeneration of di-tin(II) cryptand complex 1.
- This article is part of the themed collection: ISACS17: Challenges in Chemical Renewable Energy


 Please wait while we load your content...
Please wait while we load your content...