Asymmetric synthesis of chiral β-alkynyl carbonyl and sulfonyl derivatives via sequential palladium and copper catalysis†
Abstract
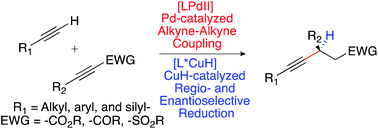
We present a full account detailing the development of a sequential catalysis strategy for the synthesis of chiral β-alkynyl carbonyl and sulfonyl derivatives. A palladium-catalyzed cross coupling of terminal alkyne donors with acetylenic ester, ketone, and sulfone acceptors generates stereodefined enynes in high yield. These compounds are engaged in an unprecedented, regio- and enantioselective copper-catalyzed conjugate reduction. The process exhibits a high functional group tolerance, and this enables the synthesis of a broad range of chiral products from simple, readily available alkyne precursors. The utility of the method is demonstrated through the elaboration of the chiral β-alkynyl products into a variety of different molecular scaffolds. Its value in complex molecule synthesis is further validated through a concise, enantioselective synthesis of AMG 837, a potent GPR40 receptor agonist.

 Please wait while we load your content...
Please wait while we load your content...