The reactivity of acyl chlorides towards sodium phosphaethynolate, Na(OCP): a mechanistic case study†
Abstract
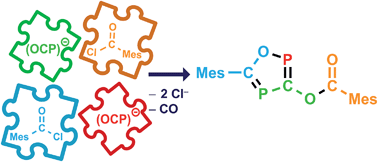
The reaction of Na(OCP) with mesitoyl chloride delivers an ester functionalized 1,2,4-oxadiphosphole in a clean and P-atom economic way. The reaction mechanism has been elucidated by means of detailed NMR-spectroscopic, kinetic and computational studies. The initially formed acyl phosphaketene undergoes a pseudo-coarctate cyclization with an (OCP)− anion under the loss of carbon monoxide to yield a five-membered ring anion. Subsequently, the nucleophilic attack of the formed heterocyclic anion on a second acyl chloride molecule results in the 1,2,4-oxadiphosphole. The transient acyl phosphaketene is conserved during the reaction in the form of four-membered ring adducts, which act as a reservoir. Consequently, the phosphaethynolate anion has three different functions in these reactions: it acts as a nucleophile, as an en-component in [2 + 2] cycloadditions and as a formal P− transfer reagent.


 Please wait while we load your content...
Please wait while we load your content...