Tandem reactions in self-sorted catalytic molecular hydrogels†
Abstract
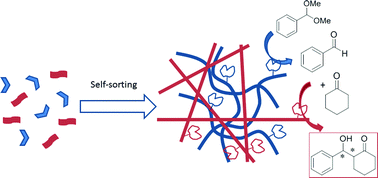
By equipping mutually incompatible carboxylic acid and proline catalytic groups with different self-assembling motives we have achieved self-sorting of the resulting catalytic gelators, namely SucVal8 and ProValDoc, into different supramolecular fibers, thus preventing the acidic and basic catalytic groups from interfering with each other. The resulting spatial separation of the incompatible catalytic functions is found to be essential to achieve one-pot deacetalization–aldol tandem reactions with up to 85% efficiency and 90% enantioselectivity. On the contrary, when SucVal8 was co-assembled with a structurally similar catalytically active hydrogelator (ProVal8), self-sorting was precluded and no tandem catalysis was observed.
- This article is part of the themed collection: ISACS18: Challenges in Organic Materials and Supramolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...