Cycloheptatrienyl trianion: an elusive bridge in the search of exchange coupled dinuclear organolanthanide single-molecule magnets†
Abstract
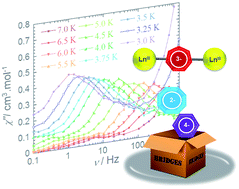
The preparation of η-cyclopentadienyl (η5-C5R5), η-arene (η6-C6R6), and η-cyclooctatetraenyl (η8-C8R8) bridging motifs are common in organometallic chemistry; however, the synthetic preparation of η-cycloheptatrienyl (η7-C7R7) bridging motifs has remained a synthetic challenge in 4f chemistry. To this end, we have developed a synthetic route towards a series of rare dinuclear organolanthanide inverse sandwich complexes containing the elusive η7-C7H7 bridge. Herein, we present the structures and magnetic properties of the lanthanide inverse sandwich complexes [KLn2(C7H7)(N(SiMe3)2)4] (Ln = GdIII (1), DyIII (2), ErIII (3)) and [K(THF)2Er2(C7H7)(N(SiMe3)2)4] (4). These compounds are the first single-molecule magnets (SMMs) to feature this type of bridging motif. Furthermore, η7-C7H7 was found to efficiently promote ferromagnetic exchange interactions between metal ions. Variable temperature dc magnetic susceptibility measurements and subsequent simulations give significant exchange constants of J = +1.384, +1.798, and +3.149 cm−1 and dipolar constants of J = −0.603, −0.601, and −0.475 cm−1 for compounds 2–4, respectively. Frequency dependent ac susceptibility measurements under an applied static field resulted in the observation of dual relaxation processes, and brought forth a greater understanding of the intermolecularly driven process at high frequency. In particular, this type of analysis of compound 3 under 800 Oe elicited an energy barrier of Ueff = 58 K. Ab initio calculations were performed in order to understand the nature of magnetic coupling and the origin of slow relaxation of magnetisation. Through these studies, the effect of the amido ancillary ligands on the magnetic axiality of the lanthanide ions was found to be competitive with the crystal field of the η7-C7H7 π-electron cloud. Our findings suggest that the tunability of the dipolar and exchange components of the magnetic interactions lie within the dihedral angle imposed by the amido ligands, thus offering potential for the development of new exchange coupled lanthanide systems.


 Please wait while we load your content...
Please wait while we load your content...