Near diffusion-controlled reaction of a Zn(Cys)4 zinc finger with hypochlorous acid†
Abstract
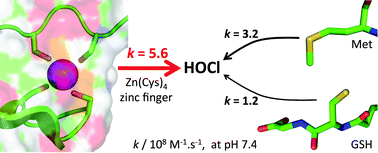
Hypochlorous acid (HOCl) is one of the strongest oxidants produced in mammals to kill invading microorganisms. The bacterial response to HOCl involves proteins that are able to sense HOCl using methionine, free cysteines or zinc-bound cysteines of zinc finger sites. Although the reactivity of methionine or free cysteine with HOCl is well documented at the molecular level, this is not the case for zinc-bound cysteines. We present here a study that aims at filling this gap. Using a model peptide of the Zn(Cys)4 zinc finger site of the chaperone Hsp33, a protein involved in the defence against HOCl in bacteria, we show that HOCl oxidation of this model leads to the formation of two disulfides. A detailed mechanistic and kinetic study of this reaction, relying on stopped-flow measurements and competitive oxidation with methionine, reveals very high rate constants: the absolute second-order rate constants for the reaction of the model zinc finger with HOCl and its conjugated base ClO− are (9.3 ± 0.8) × 108 M−1 s−1 and (1.2 ± 0.2) × 104 M−1 s−1, the former approaching the diffusion limit. Revised values of the second-order rate constants for the reaction of methionine with HOCl and ClO− were also determined to be (5.5 ± 0.8) × 108 M−1 s−1 and (7 ± 5) × 102 M−1 s−1, respectively. At physiological pH, the zinc finger site reacts faster with HOCl than methionine and glutathione or cysteine. This study demonstrates that zinc fingers are potent targets for HOCl and confirms that they may serve as HOCl sensors as proposed for Hsp33.

 Please wait while we load your content...
Please wait while we load your content...