Indacenodibenzothiophenes: synthesis, optoelectronic properties and materials applications of molecules with strong antiaromatic character
Abstract
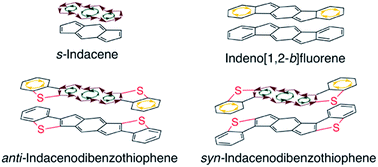
Indeno[1,2-b]fluorenes (IFs), while containing 4n π-electrons, are best described as two aromatic benzene rings fused to a weakly paratropic s-indacene core. In this study, we find that replacement of the outer benzene rings of an IF with benzothiophenes allows the antiaromaticity of the central s-indacene to strongly reassert itself. Herein we report a combined synthetic, computational, structural, and materials study of anti- and syn-indacenodibenzothiophenes (IDBTs). We have developed an efficient and scalable synthesis for preparation of a series of aryl- and ethynyl-substituted IDBTs. NICS-XY scans and ACID calculations reveal an increasingly antiaromatic core from [1,2-b]IF to anti-IDBT, with syn-IDBT being nearly as antiaromatic as the parent s-indacene. As an initial evaluation, the intermolecular electronic couplings and electronic band structure of a diethynyl anti-IDBT derivative reveal the potential for hole and/or electron transport. OFETs constructed using this molecule show the highest hole mobilities yet achieved for a fully conjugated IF derivative.

 Please wait while we load your content...
Please wait while we load your content...