Nickel-catalyzed enantioselective arylation of pyridine†
Abstract
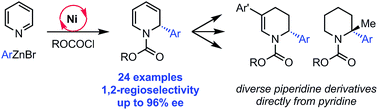
We report an enantioselective Ni-catalyzed cross coupling of arylzinc reagents with pyridinium ions formed in situ from pyridine and a chloroformate. This reaction provides enantioenriched 2-aryl-1,2-dihydropyridine products that can be elaborated to numerous piperidine derivatives with little or no loss in ee. This method is notable for its use of pyridine, a feedstock chemical, to build a versatile, chiral heterocycle in a single synthetic step.

 Please wait while we load your content...
Please wait while we load your content...