Reversible gel–sol photoswitching with an overcrowded alkene-based bis-urea supergelator†
Abstract
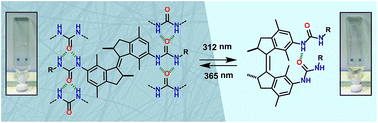
A new type of low-molecular-weight gelator (LMWG), i.e. overcrowded alkene-based bis-ureas, can be switched effectively between cis and trans isomers using light as demonstrated by 1H NMR and UV-Vis spectroscopy. Gelation studies reveal that one of the synthesized trans compounds forms stable gels in aromatic hydrocarbon solvents down to a critical concentration of 0.4 mg mL−1. Transmission electron microscopy (TEM) shows that this gel consists of an entangled fibrous network. For the trans isomer of this LMWG intermolecular urea hydrogen bonding is observed in the solid state, whereas density functional theory (DFT) geometry optimization of the cis isomer indicates the possible formation of an intramolecular hydrogen bond. Irradiation of the gel triggers trans-to-cis isomerization and consequently, a gel–sol phase transition. This process can be fully reversed by altering the irradiation wavelength.

 Please wait while we load your content...
Please wait while we load your content...