Negative ion photoelectron spectroscopy of P2N3−: electron affinity and electronic structures of P2N3˙†
Abstract
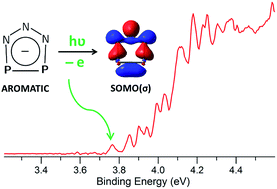
We report here a negative ion photoelectron spectroscopy (NIPES) and ab initio study of the recently synthesized planar aromatic inorganic ion P2N3−, to investigate the electronic structures of P2N3− and its neutral P2N3˙ radical. The adiabatic detachment energy of P2N3− (electron affinity of P2N3˙) was determined to be 3.765 ± 0.010 eV, indicating high stability for the P2N3− anion. Ab initio electronic structure calculations reveal the existence of five, low-lying, electronic states in the neutral P2N3˙ radical. Calculation of the Franck–Condon factors (FCFs) for each anion-to-neutral electronic transition and comparison of the resulting simulated NIPE spectrum with the vibrational structure in the observed spectrum allows the first four excited states of P2N3˙ to be determined to lie 6.2, 6.7, 11.5, and 22.8 kcal mol−1 above the ground state of the radical, which is found to be a 6π-electron, 2A1, σ state.
- This article is part of the themed collection: ISACS17: Challenges in Chemical Renewable Energy


 Please wait while we load your content...
Please wait while we load your content...