Chiral metal–macrocycle frameworks: supramolecular chirality induction and helicity inversion of the helical macrocyclic structures†
Abstract
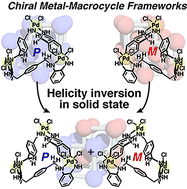
Porous molecular solids composed of discrete macrocycles/cages have great potential for catalysis, separation and sensing techniques. Dynamic structural transformation of the host building blocks, especially a helicity inversion responsive to chemical triggers, is central to upgrading the spatial functions. Here we have achieved the syntheses of homochiral porous molecular solids composed of helical metal macrocycles through supramolecular chirality induction to both enantiomorphic forms with the aid of two different enantiopure sugar-derived lactones in the crystallization process. Moreover, we found that the helicity of the macrocyclic skeletons can be inverted in the crystalline state only by changing the type of solvent. This finding would lead to dynamic control of space chirality in connection with optical resolution, chiral amplification and asymmetric reactions.

 Please wait while we load your content...
Please wait while we load your content...