Stabilising the lowest energy charge-separated state in a {metal chromophore – fullerene} assembly: a tuneable panchromatic absorbing donor–acceptor triad†
Abstract
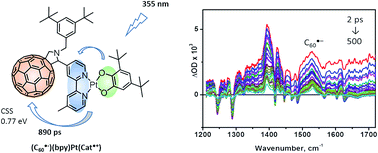
Photoreduction of fullerene and the consequent stabilisation of a charge-separated state in a donor–acceptor assembly have been achieved, overcoming the common problem of a fullerene-based triplet state being an energy sink that prevents charge-separation. A route to incorporate a C60-fullerene electron acceptor moiety into a catecholate-Pt(II)-diimine photoactive dyad, which contains an unusually strong electron donor, 3,5-di-tert-butylcatecholate, has been developed. The synthetic methodology is based on the formation of the aldehyde functionalised bipyridine-Pt(II)-3,5-di-tert-butylcatechol dyad which is then added to the fullerene cage via a Prato cycloaddition reaction. The resultant product is the first example of a fullerene-diimine-Pt-catecholate donor–acceptor triad, C60bpy-Pt-cat. The triad exhibits an intense solvatochromic absorption band in the visible region due to catechol-to-diimine charge-transfer, which, together with fullerene-based transitions, provides efficient and tuneable light harvesting of the majority of the UV/visible spectral range. Cyclic voltammetry, EPR and UV/vis/IR spectroelectrochemistry reveal redox behaviour with a wealth of reversible reduction and oxidation processes forming multiply charged species and storing multiple redox equivalents. Ultrafast transient absorption and time resolved infrared spectroscopy, supported by molecular modelling, reveal the formation of a charge-separated state [C60˙−bpy-Pt-cat˙+] with a lifetime of ∼890 ps. The formation of cat˙+ in the excited state is evidenced directly by characteristic absorption bands in the 400–500 nm region, while the formation of C60˙− was confirmed directly by time-resolved infrared spectroscopy, TRIR. An IR-spectroelectrochemical study of the mono-reduced building block (C60-bpy)PtCl2, revealed a characteristic C60˙− vibrational feature at 1530 cm−1, which was also detected in the TRIR spectra. This combination of experiments offers the first direct IR-identification of C60˙− species in solution, and paves the way towards the application of transient infrared spectroscopy to the study of light-induced charge-separation in C60-containing assemblies, as well as fullerene films and fullerene/polymer blends in various OPV devices. Identification of the unique vibrational signature of a C60-anion provides a new way to follow photoinduced processes in fullerene-containing assemblies by means of time-resolved vibrational spectroscopy, as demonstrated for the fullerene-transition metal chromophore assembly with the lowest energy charge-separated excited state.
- This article is part of the themed collection: Celebrating the 2017 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...