Minimization of dynamic effects in the evolution of dihydrofolate reductase†
Abstract
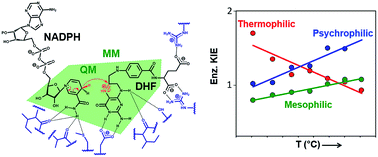
Protein isotope labeling is a powerful technique to probe functionally important motions in enzyme catalysis and can be applied to investigate the conformational dynamics of proteins. Previous investigations have indicated that dynamic coupling is detrimental to catalysis by dihydrofolate reductase (DHFR) from the mesophile Escherichia coli (EcDHFR). Comparison of DHFRs from organisms adapted to survive at a wide range of temperatures suggests that dynamic coupling in DHFR catalysis has been minimized during evolution; it arises from reorganizational motions needed to facilitate charge transfer events. Contrary to the behaviour observed for the DHFR from the moderate thermophile Geobacillus stearothermophilus (BsDHFR), the chemical transformation catalyzed by the cold-adapted bacterium Moritella profunda (MpDHFR) is only weakly affected by protein isotope substitutions at low temperatures, but the isotopically substituted enzyme is a substantially inferior catalyst at higher, non-physiological temperatures. QM/MM studies revealed that this behaviour is caused by the enzyme’s structural sensitivity to temperature changes, which enhances unfavorable dynamic coupling at higher temperatures by promoting additional recrossing trajectories on the transition state dividing surface. We postulate that these motions are minimized by fine-tuning DHFR flexibility through optimization of the free energy surface of the reaction, such that a nearly static reaction-ready configuration with optimal electrostatic properties is maintained under physiological conditions.


 Please wait while we load your content...
Please wait while we load your content...