Rh(iii)-catalyzed diastereoselective C–H bond addition/cyclization cascade of enone tethered aldehydes†
Abstract
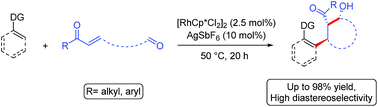
The Rh(III)-catalyzed cascade addition of a C–H bond across alkene and carbonyl π-bonds is reported. The reaction proceeds under mild reaction conditions with low catalyst loading. A range of directing groups were shown to be effective as was the functionalization of alkenyl in addition to aromatic C(sp2)–H bonds. When the enone and aldehyde electrophile were tethered together, cyclic β-hydroxy ketones with three contiguous stereocenters were obtained with high diastereoselectivity. The intermolecular three-component cascade reaction was demonstrated for both aldehyde and imine electrophiles. Moreover, the first X-ray structure of a cationic Cp*Rh(III) enolate with interatomic distances consistent with an η3-bound enolate is reported.
- This article is part of the themed collection: Top 50 Articles of 2016: Organic Chemistry

 Please wait while we load your content...
Please wait while we load your content...