Combining photochemistry and catalysis: rapid access to sp3 – rich polyheterocycles from simple pyrroles†
Abstract
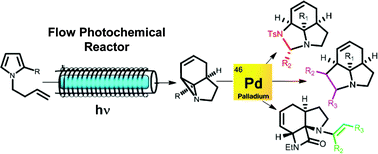
Use of FEP flow reactor technology allows access to gram quantities of photochemically-generated tricyclic aziridines. These undergo a range of novel palladium-catalyzed ring-opening and cycloaddition reactions, likely driven by their inherent strain, allowing incorporation of further functionality by fusing additional heterocyclic rings onto these already complex polycyclic cores. This rapid, 2-step access to complex sp3 – rich heterocycles should be of interest to those in the fields of drug discovery and natural product synthesis.

 Please wait while we load your content...
Please wait while we load your content...