Abstract
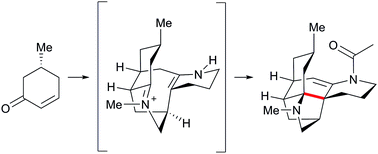
(+)-Fastigiatine was assembled in six steps from (R)-5-methylcyclohex-2-en-1-one. Intermolecular Diels–Alder reaction introduced most of the carbon atoms for the target. The two Boc-protected nitrogen atom building blocks were introduced by a Suzuki coupling and a cuprate addition. A biomimetic transannular Mannich reaction generated the two quaternary centers at a late stage. Each step builds core bonds, and combined with a minimalist protecting group strategy, this approach led to a very concise synthesis.


 Please wait while we load your content...
Please wait while we load your content...