Oxidation triggers extensive conjugation and unusual stabilization of two di-heme dication diradical intermediates: role of bridging group for electronic communication†
Abstract
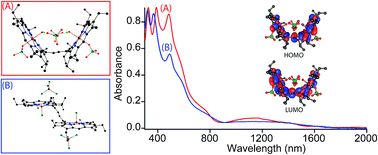
MauG is a diheme enzyme that utilizes two covalently bound c-type hemes to catalyse the biosynthesis of the protein-derived cofactor tryptophan tryptophylquinone. The two hemes are physically separated by 14.5 Å and a hole-hopping mechanism is proposed in which a tryptophan residue located between the hemes undergoes reversible oxidation and reduction to increase the effective electronic coupling element and enhance the rate of reversible electron transfer between the hemes in bis-Fe(IV) MauG. The present work describes the structure and spectroscopic investigation of 2e-oxidations of the synthetic diheme analogs in which two heme centers are covalently connected through a conjugated ethylene bridge that leads to the stabilization of two unusual trans conformations (U and P′ forms) with different and distinct spectroscopic and geometric features. Unlike in MauG, where the two oxidizing equivalents are distributed within the diheme system giving rise to the bis-Fe(IV) redox state, the synthetic analog stabilizes two ferric hemes, each coupled with a porphyrin cation radical, a scenario resembling the binuclear dication diradical complex. Interestingly, charge resonance-transition phenomena are observed here both in 1e and 2e-oxidised species from the same system, which are also clearly distinguishable by their relative position and intensity. Detailed UV-vis-NIR, X-ray, Mössbauer, EPR and 1H NMR spectroscopic investigations as well as variable temperature magnetic studies have unraveled strong electronic communications between two porphyrin π-cation radicals through the bridging ethylene group. The extensive π-conjugation also allows antiferromagnetic coupling between iron(III) centers and porphyrin radical spins of both rings. DFT calculations revealed extended π-conjugation and H-bonding interaction as the major factors in controlling the stability of the conformers.

 Please wait while we load your content...
Please wait while we load your content...