Thermodynamic synthesis of solution processable ladder polymers†
Abstract
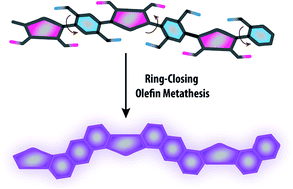
The synthesis of a carbazole-derived, well-defined ladder polymer was achieved under thermodynamic control by employing reversible ring-closing olefin metathesis. This unique approach featured mild conditions and excellent efficiency, affording the ladder polymer backbone with minimum levels of unreacted defects. Rigorous NMR analysis on a 13C isotope-enriched product revealed that the main-chain contained less than 1% of unreacted precursory vinyl groups. The rigid conformation of the ladder-type backbone was confirmed by photophysical analysis, while the extended rod-like structure was visualized under scanning tunneling microscope. Excellent solubility of this polymer in common organic solvents allowed for feasible processing of thin films using solution-casting techniques. Atomic force microscopy and grazing incident X-ray scattering revealed a uniform and amorphous morphology of these films, in sharp contrast to the polycrystalline thin films of its small molecular counterpart.

 Please wait while we load your content...
Please wait while we load your content...