Low-temperature fabrication of lithium aluminum oxide phosphate solid electrolyte thin films from aqueous precursors†
Abstract
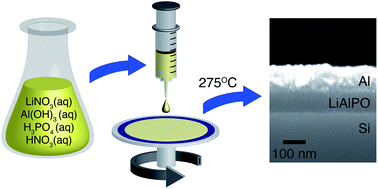
Low-temperature routes to solid electrolytes are important for construction of solid-state batteries, electrochromic devices, electrolyte-gated transistors, high-energy capacitors and sensors. Here we report an environmentally friendly aqueous solution route to amorphous thin films of the solid electrolyte lithium aluminum oxide phosphate (LiAlPO). This route allows production of high quality films at very low temperatures (275 °C), 600 °C lower than traditional melt quenching routes to LiAlPO. Films of thicknesses ranging from 20–150 nm produced by this route are extremely smooth and fully dense, with temperature dependent conductivities similar to those reported for samples made by melt quenching techniques. The average room temperature conductivity of LiAlPO films was measured to be σDC = 2.6 × 10−8 S cm−1. Film evolution was monitored by TGA-DSC and FTIR, and resulting films were characterized using FTIR, XPS, SEM, and XRD. These techniques indicate that water and nitrate removal is complete by 250 °C, and the films remain amorphous to 400 °C. The approach developed demonstrates a simple, inexpensive and environmentally benign deposition route for the fabrication of inorganic solid electrolyte thin films, using LiAlPO as a model system.

 Please wait while we load your content...
Please wait while we load your content...