Synthesis of 13N-labelled polysubstituted triazoles via Huisgen cycloaddition†
Abstract
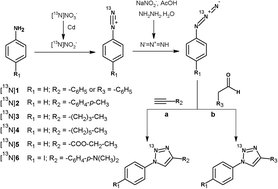
The use of the positron emitter nitrogen-13 (13N) has been historically restricted due to its short half-life (T1/2 = 9.97 min). However, its stable isotopes (nitrogen-14 and nitrogen-15) are present in many biologically active molecules; therefore, the incorporation of 13N in the toolbox of PET chemists might be a valuable option for the preparation of new labelled compounds or incorporation of the label in different positions. Here we present the unprecedented radiosynthesis of 13N-labelled polysubstituted triazoles via Huisgen cycloaddition by reaction of 13N-labelled aromatic azides with alkyne derivatives and aldehydes. Six different 13N-labelled triazoles were successfully synthesized. After automatization of the synthetic process and optimization of experimental conditions, one selected triazole could be prepared with high radiochemical purity and decay-corrected radiochemical yields of 11 ± 2%. The amount of activity obtained should be sufficient to approach future in vitro and in vivo studies. The novel methodology might open new avenues for the preparation of radiotracers which cannot be labelled using other more conventional positron emitters.

 Please wait while we load your content...
Please wait while we load your content...