Crystal-to-crystal photo-reversible polymerization mechanism of bis-thymine derivative†
Abstract
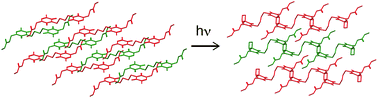
Solid-state photo-polymerization in crystals can produce stereoregular polymer molecules in environmentally friendly solvent-free systems. The polymerization mechanism of bis-thymine derivatives, such as dimethyl-3,3′-(3,3′-(butane-1,4-diyl)bis(5-methyl-2,4-dioxo-3,4-dihydropyrimidine-3,1(2H)-diyl))dipropanoate (1), known as unique molecules that can topochemically and reversibly polymerize in the crystalline state via [2 + 2]-cycloaddition reactions upon UV irradiation, remained to be solved. In this manuscript, the crystal structure of the polymeric photoproduct (1P) from a bis-thymine derivative 1 was determined using ab initio powder X-ray diffraction data and applied to investigate the polymerization mechanism of bis-thymine derivatives. The topochemical polymerization was found to be achieved via [2 + 2]-cycloaddition with the flexible butyl chain between thyminyl rings relieving the distortion of whole structure derived from cyclobutane formation. The crystal structure of 1P also showed that it polymerized stereoregularly with trans–anti cyclobutane conformations.


 Please wait while we load your content...
Please wait while we load your content...