Synthesis and anti-proliferative evaluation of novel 3,4-dihydro-2H-1,3-oxazine derivatives of bakuchiol†‡
Abstract
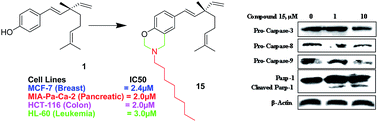
A new series of 3,4-dihydro-2H-1,3-oxazine derivatives of bakuchiol 1 was synthesized through the Mannich-type condensation–cyclization reaction of 1 with formaldehyde and appropriate primary amines. On cytotoxicity evaluation against a panel of four human cancer cell lines, most of the derivatives showed a higher cytotoxic profile than the parent molecule. The best results were observed for compound 15 with IC50 values of 2, 2, 2.4 and 3 μM against MIA-Pa-Ca-2, HCT-116, MCF-7 and HL-60 cells, respectively. A mechanistic study of compound 15 revealed that it caused a loss in the mitochondrial membrane potential in a concentration-dependent manner, accompanied by the activation of caspase-9 and -3, which cleave PARP-1. It also activated caspase-8, which is involved in the extrinsic apoptotic pathway. Therefore, we demonstrated that it induces apoptosis via both intrinsic and extrinsic pathways in human pancreatic cancer MIA-Pa-Ca-2 cells.

 Please wait while we load your content...
Please wait while we load your content...